Research Questions
In the Sipe laboratory, we study the interactions between neurons and glia ('non-neuronal cells') in sensory processing, neuromodulation, stress, and addiction. This research can be divided into the following groups:
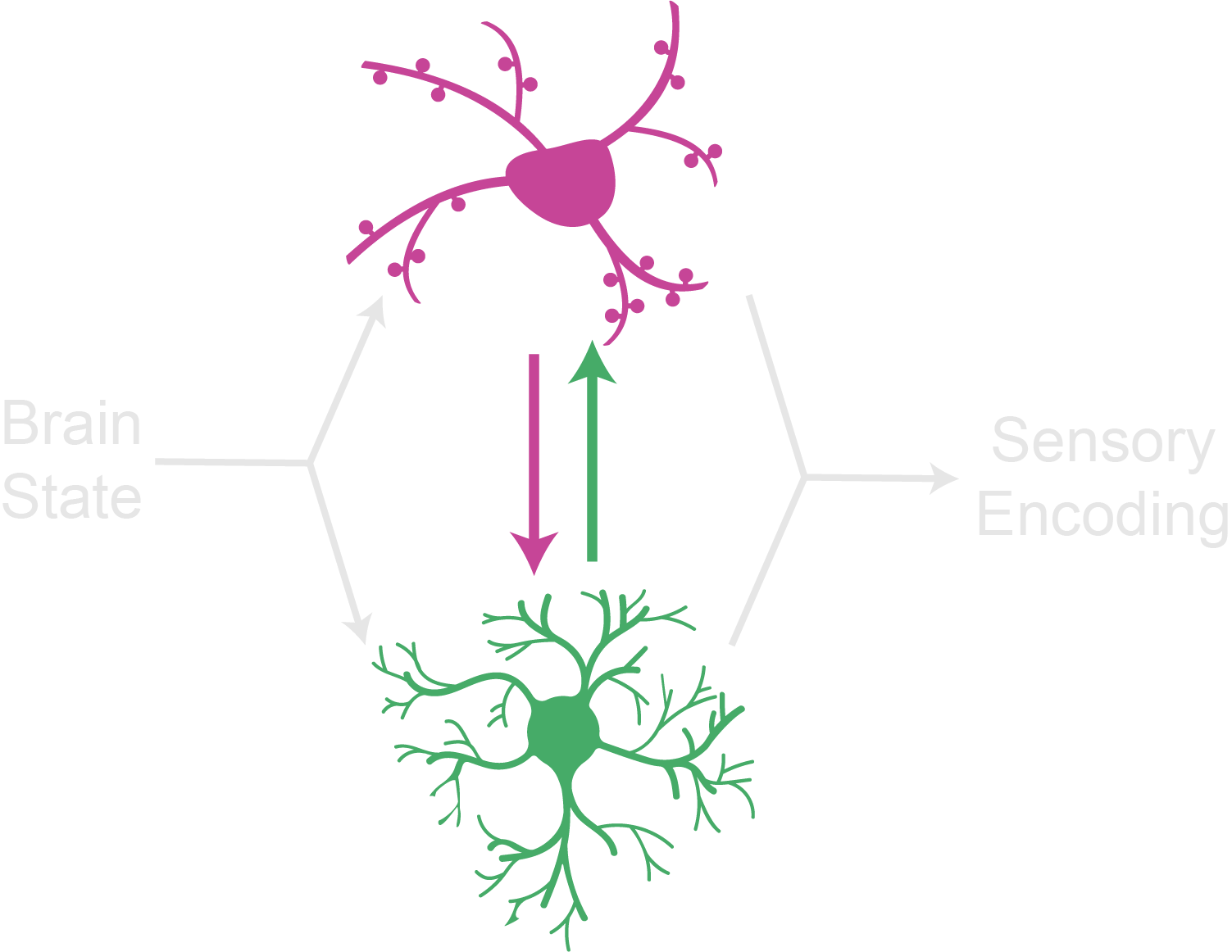
Astrocyte-neuron communication in adaptive sensory encoding
Brain state shifts fundamentally shape sensory processing and adaptive decision-making. How the brain flexibly shifts sensory encoding in response to changing goal-directed behavior and external contexts remains poorly understood. Our laboratory investigates bidirectional astrocyte-neuron communication in brain state-dependent sensory encoding, particularly visual encoding in a virtual foraging task.Neuromodulators and astrocytes in contextual learning
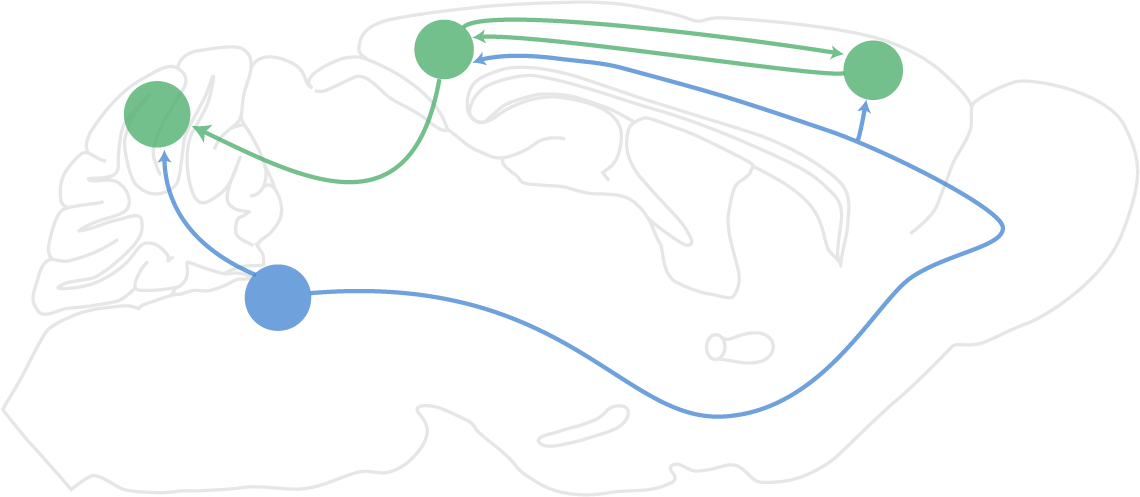
The brain must constantly switch between executing behavior in learned contexts and integrating sensory information to learn new contexts. How neuromodulators such as norepinephrine signal to both astrocytes and neurons to coordinate plasticity and learning across multiple brain regions is not well known. Our laboratory investigates these dynamics in models of learning, especially the conditioned eyeblink paradigm.Brain states underlying alcohol as a self-medication for stress
Stress-linked disorders such as anxiety, depression and post-traumatic stress disorder (PTSD) have a high comorbidity with substant use disorders, especially alcohol use disorder. This suggests that alcohol may initially serve as a self-medication for stress-linked symptoms before descending into addiction. How alcohol initially has ameliorative effects on brain states related to stress is not well known. Our laboratory investigates this question using a mouse model of binge-like alcohol consumption to determine the cellular mechanisms underlying brain states predictive of alcohol consumption.Brain-body allostasis in stress acclimation
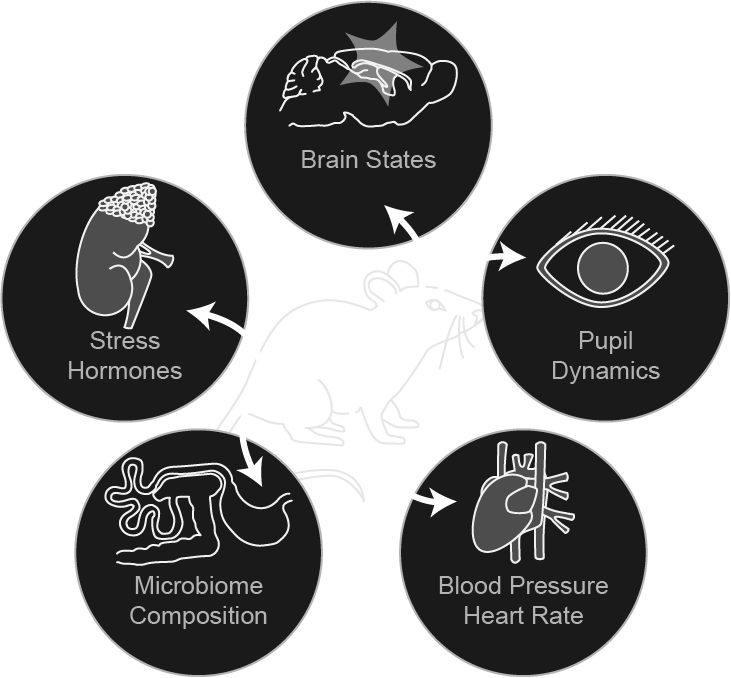
The brain and body must often coordinate physiological shifts to new set points in response to homeostatic perturbations, a process termed allostasis. As an organ, the brain must bidirectionally communication with other organs in the body to synchronize physiological states in response to allostatic loads such as psychological stress. How brain states map onto whole-body physiology is largely unknown. Our laboratory investigates brain-body allostasis in a head-fixed mouse model of stress acclimation.